Research
Our research group investigates the biosynthesis and function of membrane lipids, storage lipids (triacylglycerol) and lipid vitamins (tocopherol/vitamin E; carotenoids/provitamin A) in model and crop plants (Arabidopsis, barley, African oil palm) and in cyanobacteria.









Professor Dr. Peter Dörmann
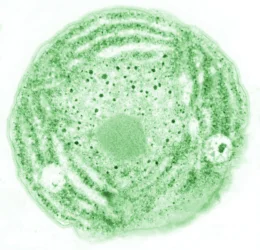
Biological membranes form the barrier between the cell and its environment, and they divide eukaryotic cells into functional compartments. Plants and many bacteria produce a unique set of glycolipids that differ from the membrane lipids in animals. Glycolipids are enriched in the chloroplast membranes of plants, where they outnumber phospholipids. The thylakoid membranes of chloroplasts harbor the photosynthetic complexes that convert light into chemical energy.
We are interested in the biosynthesis of the glycolipid digalactosyldiacylglycerol (DGDG), which is produced by DGDG synthases from monogalactosyldiacylglycerol (MGDG) (Dörmann et al., 1999). Arabidopsis thaliana contains two DGDG synthases, DGD1 and DGD2. The corresponding single and double mutants are shown in Figure 1 (Kelly et al., 2003). We study the biosynthesis and function of DGDG in plants.


Professor Dr. Peter Dörmann
Tocopherol (vitamin E) is a lipid antioxidant that contributes to the degradation of reactive oxygen species in plants and animals. A tocopherol-deficient mutant of Arabidopsis identified in our laboratory, vitamin E-deficient mutant 1 (vte1), is defective in tocopherol cyclase and completely devoid of tocopherol (Porfirova et al., 2002). Tocopherol deficiency causes oxidative stress in the thylakoid membranes of chloroplasts.
The decrease in tocopherol can be compensated by zeaxanthin, a carotenoid with antioxidant activity involved in the protection of photosynthetic complexes against high light (Havaux et al., 2005). Tocopherol is synthesized from phytyl-diphosphate and homogentisate in the chloroplasts of plants. In the past years, it became clear that phytol for tocopherol synthesis is mostly derived from chlorophyll breakdown rather than from direct reduction of geranylgeranyl-diphosphate derived from the chloroplast MEP pathway. In line with this scenario, the VTE5 gene encoding phytol kinase has previously been described.
We identified VTE6, another gene involved in tocopherol synthesis, which converts phytyl-phosphate into phytyl-diphosphate (vom Dorp et al., 2015).
Professor Dr. Peter Dörmann
This project is funded by the DFG
The African oil palm (Elaeis guineensis) is the most important oil crop in the world. The crude palm oil is extracted from the mesocarp tissue of the fruits. Because of the high content in carotenes (provitamin A), the oil is prone to oxidation and has a limited shelf life.
The palm oil also contains tocochromanols (vitamin E), a natural antioxidant, but its content is insufficient to protect the oil against oxidation. Together with our collaboration partners in Ghana and Cameroon, we have screened populations of wild palm trees from West Africa for palm oils with a superior composition of provitamin A and vitamin E.
The goal of this project is to identify trees that produce a crude palm oil with higher stability and longer shelf life. The corresponding traits can then be introgressed into elite breeding material to obtain trees that produce an oil with longer shelf life that can be transported to distal markets and employed to fight vitamin A deficiency in Africa.


Professor Dr. Peter Dörmann
This project is funded by the DFG
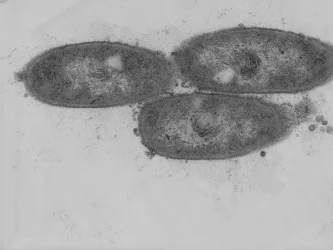
Cyanobacteria are bacteria that, like plants and algae, carry out oxygenic photosynthesis. As prokaryotic cells, their cell structure is less complex. We identified the first gene of oil (triacylglycerol) synthesis in cyanobacteria (Aizouq et al., 2020). The elucidation of the biosynthetic pathway for triacylglycerol in cyanobacteria enables new strategies for biotechnological oil production in microorganisms.
Cyanobacteria are amenable to molecular modifications such as transformation to generate knock-out mutations or overexpression of genes. Cyanobacteria are therefore ideal for the biotechnological production of oil as a renewable resource.
Professor Dr. Peter Dörmann
This project is funded by the BMBF
Alcanivorax borkumensis is a marine bacterium that can employ hydrocarbons, e.g. released during petroleum spills in the oceans, as sole carbon source. The bacterium produces a surfactant, i.e. a glycine-glucolipid consisting of four 3-hydroxy fatty acids bound to a glucose in glycosidic linkage and to a glycine in amide linkage (Cui et al., 2022).
We are interested in the biosynthesis and function of the A. borkumensis surfactant during the metabolization of hydrocarbons.


Professor Dr. Peter Dörmann
This project is funded by the Cluster of Excellence 'PhenoRob'
Phosphate is one of the most important macronutrients for plants. We study the biochemical and physiological responses of barley (Hordeum vulgare) to phosphate deprivation. To this end, the changes in metabolites in different plant organs are recorded.
We generate knock-out barley mutants of genes involved in phosphate sensing, and we produce transgenic barley plants expressing reporter genes. These lines will be instrumental to train neural networks for the identification of early symptoms of phosphate deprivation.
Professor Dr. Peter Dörmann and Priv.-Doz. Dr. Margot Schulz
This project is funded by the DFG priority program DECRyPT
Plant specialized (secondary) metabolites including benzoxazolinone (BOA), gramine and quercetin are produced by different plant species, including barley (Hordeum vulgare) and Lotus japonicus.
We are investigating the influence of these three metabolites on bacterial community structure in agricultural soils. The input of plant metabolites to the soil alters the abundance of many bacterial genera (Schütz et al., 2021). More than 110 bacterial strains were isolated and cultured after treatment with the plant metabolites. The strains have been tested individually for their tolerance to BOA, gramine or quercetin.
We are currently searching for bacterial genes involved in the degradation of the metabolites, and we study the structure of the degradation products of the plant metabolites including their effects on Arabidopsis.

Dr. Georg Hölzl
Similar to plants, some bacteria can alter their membrane lipid composition by producing glycolipids and other non-phosphorous lipids under phosphate deficiency. These lipids include galactolipids, glucolipids, betaine lipids (DGTS, diacylglyceroltrimethylhomoserine), and ornithine lipid.
We have discovered new forms of glycolipids and ornithine lipid in Agrobacterium tumefaciens (a plant pathogenic bacterium used for plant transformation) (Geske et al., 2013; Semeniuk et al., 2014) and in Mesorhizobium loti (nodule bacterium of Lotus japonicus). (Devers et al., 2011).
We have tested additional genes involved in the regulation of lipid synthesis in these and other bacteria using knock-out mutant strategies.


Dr. Georg Hölzl and Professor Dr. Peter Dörmann
Plant oils represent a major source for carbon and energy for human nutrition and contain many important lipids such as vitamins and essential fatty acids. Rapeseed (Brassica napus), a close relative of Arabidopsis thaliana, is the major oil crop in Central Europe.
Additional oil crops, e.g. Camelina sativa, are becoming more and more important as a renewable resource. Plant oil is used for human nutrition, for industrial applications (lubricants, surface finishing) as well as biofuel/jetfuel for the car and airplane business. Camelina seed contain considerable amounts of glucosinolates, specialized (secondary) plant metabolites which are unwanted in the food and feed industry.
Using genome editing (CRISPR-Cas9) of several loci involved in glucosinolate synthesis, we generated Camelina mutant lines deficient in glucosinolate production with a superior quality for food and feed production (Hölzl and Dörmann, 2023).
Dr. Katharina Gutbrod
Fatty acid phytyl esters are lipids produced in chloroplasts during stress. Like tocopherol, fatty acid phytyl esters are synthesized from phytol, a chlorophyll-derived long-chain alcohol (Ischebeck et al., 2006). Fatty acid phytyl esters accumulate only during stress and senescence in plants and temporarily store fatty acids and phytol (Lippold et al., 2012). Two enzymes, PES1 and PES2, contribute to fatty acid phytyl ester synthesis in Arabidopsis.
We study the role of these two enzymes for the synthesis of phytyl esters and other neutral lipids in chloroplasts.

Plant biochemistry and molecular allelopathy

Priv.-Doz. Dr. Margot Schulz
BOA/MBOA represent compounds derived from the benzoxazinones DIBOA/DIMBOA, secondary products of several cereals such as corn or rye, and several other species. The parent compounds may be actively released into the environment by root exudates or by decaying plants. Most compounds are bioactive. They can be modified after uptake by plants and are transformed and degraded by microorganisms that colonize soil and roots, and the products can affect the plant transcriptome and defense strategies.
Priv.-Doz. Dr. Margot Schulz
Since allelopathy is believed to contribute to the sustainability of agroecosystems, methods for allelopathic weed control and the study of positive allelopathic interactions between species are being explored for use in agricultural crop systems.
Rye mulch: In collaboration with the Institute of Agronomy, Plant Physiology and Field Crops of Università Cattolica del Sacro Cuore, the importance of BOA detoxification for the survival of insensitive weeds in environments enriched with this allelic chemical is currently being studied. Detoxification strategies explain why mulches of suitable rye cultivars containing benzoxazinoids and flavonoids are selectively useful for weed control in organic farming systems.
Terpenoids to promote plant growth. In another approach, the use of natural mixtures of volatiles and defined monoterpenes for dose-dependent growth promotion of cabbage seedlings is being investigated in comparison with the model Brassicacea Arabidopsis thaliana. The research is carried out in cooperation with Prof. Andreas Ulbrich, Osnabrück University of Applied Sciences.